electronic configuration of cu|Electron Configuration for Cu, Cu+, and Cu2+ (Copper and : Tagatay Learn how to write the electron configuration for copper and its ions using the periodic table or an electron configuration chart. See the correct and incorrect ways to arrange the electrons in orbitals and the exceptions for copper. Week 11 of the 2023 NFL season wraps up Monday night with a huge matchup as the Kansas City Chiefs (7-2) host the Philadelphia Eagles (8-1).. What can we expect from a betting standpoint? Betting .
PH0 · Writing the Electron Configuration for Copper (Cu)
PH1 · What is the correct electronic configuration of Copper Cu?
PH2 · Electron Configuration for Cu, Cu+, and Cu2+ (Copper and
PH3 · Electron Configuration for Cu, Cu+, and Cu2+ (Copper and
PH4 · Electron Configuration for Copper (Cu, Cu+, Cu2+)
PH5 · Electron Configuration for Copper (Cu, Cu+, Cu2+)
PH6 · Electron Configuration For Copper
PH7 · Electron Configuration Chart of All Elements (Full Chart)
PH8 · Copper Electron Configuration (Cu) with Orbital Diagram
PH9 · Copper
PH10 · 1.9: Electron Configurations for Transition Metal Elements
PH11 · 1.9: Electron Configurations for Transition Metal
KantotVids are collections of Pinay Porn Site videos. Who has a Viral Pinay Sex Videos, Pinay Sex Scandal and Most popular Pinay Celebrity's hot Porn Movies
electronic configuration of cu*******Learn how to write the electron configuration for copper and its ions using the periodic table or an electron configuration chart. See the correct and incorrect ways to arrange the electrons in orbitals and the exceptions for copper.
Electron Configuration for Cu, Cu+, and Cu2+ (Copper and In order to write the Calcium electron configuration we first need to know the .In order to write the Mg electron configuration we first need to know the .
When we write the configuration we'll put all 19 electrons in orbitals around the .
In order to write the Silicon electron configuration we first need to know the .Chlorine (Cl) - Electron Configuration for Copper (Cu, Cu+, Cu2+) - UMDLithium is the third element with a total of 3 electrons. In writing the electron .
electronic configuration of cu Electron Configuration for Cu, Cu+, and Cu2+ (Copper and Hul 8, 2019 — To write the configuration for the Copper ions, first we need to write the electron configuration for just Copper (Cu). We first need to find the number of .119 rows — Mar 23, 2023 — Electron configuration chart of all Elements .Learn the correct electronic configuration of copper (Cu) with an explanation and a solution. Find out why copper has a stable 3d10 configuration and how to write the electron .
Learn how to write the electron configuration of copper (Cu) and its ions (Cu+, Cu2+) using the Bohr model and the Aufbau principle. See the orbit and orbital dia.Ago 28, 2023 — Learn how to write electron configurations for transition metal elements, including copper (Cu), which has a fully-filled 3d subshell. See examples, diagrams, and exceptions to the order of filling orbitals.
Peb 26, 2012 — Learn how to write the electron configuration and orbital notation for copper (Cu) with Mr. Causey's video tutorial. See examples, tips and tricks for transition metals and orbital overlapping.
Ene 26, 2021 — What is The Electron Configuration of Copper. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of Cu. If the general pattern of filling electron orbitals is followed, then copper’s electron .Nob 13, 2020 — The electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Knowledge of the electron configuration of different atoms is .
Learn how copper atoms arrange their electrons across different shells and subshells, and how this affects its valency and properties. Find out the applications and importance of .
Hun 30, 2023 — Filling Transition Metal Orbitals. The electron configuration for the first row transition metals consists of 4s and 3d subshells with an argon (noble gas) core. This only applies to the first .Hun 20, 2023 — The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between .
The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers .
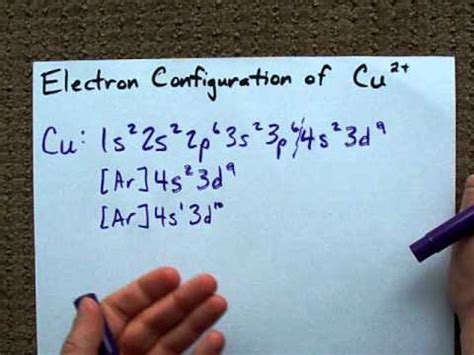
Ene 25, 2014 — Copper is in the ninth column of the transition metals in the d block of the fourth energy level of the periodic table.This would make the electron configuration for copper, #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^9# or in noble gas configuration [Ar] #4s^2 3d^9#.. However, because the 3d orbital is so much larger then the 4s orbital and the 3d .Figure \(\PageIndex{6}\): This periodic table shows the electron configuration for each subshell. By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table.
In this periodic table the electronic configuration of Copper (Cu) is wrong. The correct configuration is Cu= [Ar] 3d9 4s2 Please correct that others are right and very useful. Reply. Mentor. October 15, 2020 at 10:03 am. This is expected that the configuration of copper is 3d94s2. However, it turns out that the 3d104s1 configuration is more .electronic configuration of cuHul 7, 2014 — Using the Aufbau principle, you would write the following electron configurations Cr = [Ar] 4s^2 3d^4 Cu = [Ar] 4s^2 3d^9 The actual electron configurations are: Cr = [Ar] 4s^1 3d^5 Cu = [Ar] 4s^1 3d^10 To understand why this occurs, it is important to realize that. 1. Completely filled sublevels are more stable than .
Nob 13, 2020 — Copper is a chemical element with atomic number 29 which means there are 29 protons and 29 electrons in the atomic structure.The chemical symbol for Copper is Cu. Electron Configuration and Oxidation States of Copper. Electron configuration of Copper is [Ar] 3d10 4s1. Possible oxidation states are +1,2. Electron ConfigurationNob 4, 2014 — Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.Electron Configuration -The Electron Configuration of an Element Describes how Electrons are Distributed in their Atomic Orbitals. In Electronic Configuration electrons are arranged in various shells, Subshell and Orbital by following certain rules. To Learn how to Write Electronic Configurations, Detailed Explanation, Filling of orbital with FAQs, Visit .This is expected that the configuration of copper is 3 d 9 4 s 2. However, it turns out that the 3 d 10 4 s 1 configuration is more stable, because that way the 3d subshell is full, which is a far more stable arrangmemnt than 3 d 9. Due to extra stability of half filled and full filled orbital, Cu have configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p .Mar 26, 2020 — The electron configurations and orbital diagrams of these four elements are: Figure \(\PageIndex{5}\): Since the core electron shells correspond to noble gas electron configurations, we can abbreviate .For example, you would expect the electron configuration of Cu to be: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 9 (paramagnetic, 1 unpaired electron) and when it loses one electron to form the Cu + with the following electron configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 8 (paramagnetic; 2 unpaired electrons).
Nob 26, 2023 — Start with the electron configuration of the neutral atom. It is sufficient to use the noble gas configuration. Fe: [Ar] 4s 2 3d 6. Then, remove (or add) electrons to reflect the oxidation state of the metal. Remember to remove electrons from the orbital with the highest principal quantum number first! Fe(III) has lost 3 electrons relative to .Similarly, the observed electron configuration of copper is [Ar] 4s 1 3d 10 instead of [Ar] s 2 3d 9. The actual electron configuration may be rationalized in terms of an added stability associated with a half-filled (ns 1, np 3, nd 5, nf 7) or filled (ns 2, np 6, nd 10, nf 14) subshell. Given the small differences between higher energy levels .Ene 30, 2023 — Electronic Structure. The electronic configuration of transition metal elements are characterized as having full outer sub-orbitals and the second outermost d sub-orbitals incompletely filled, with the exception of Copper which loses one 4s orbital electron to the 3d sub-orbital for increased stability. The electron configuration for .Ago 14, 2020 — The electron configurations and orbital diagrams of these four elements are: Figure \(\PageIndex{5}\): Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core electron configuration, along with the valence electrons in a .

Hun 30, 2023 — The key thing to remember about electronic configuration is that the most stable noble gas configuration is ideal for any atom. Forming bonds are a way to approach that configuration. In particular, the transition metals form more lenient bonds with anions, cations, and neutral complexes in comparison to other elements.
You’ve just discovered the biggest free online slots library available in Canada. Like thousands of Canadian players who use VegasSlotsOnline.com every day, you now have instant access to over 16,000 free online slots that you can play right here.. You can play our free slot games from anywhere, as long as you’re connected to the internet.
electronic configuration of cu|Electron Configuration for Cu, Cu+, and Cu2+ (Copper and